Featured image from Kelly Brogan
As much as you may want to, you can’t escape germs. In fact, it’s probably better if you don’t. Humans are populated by a rich community of about 100 trillion microbes, 10 times as many cells as we have in our bodies, and weighing an estimated 3 pounds (the weight discrepancy is due to the larger size of human cells). This “microbiome” provides services from digestion to defense against pathogens, and is now considered to be as vital to good health as our other organs. Inside and out, our bodies are colonized by bacteria that perform many useful functions that we have just started to understand.

They are EVERYWHERE! Source: Nature
Digestion
Most of our microbial inhabitants live in our guts, where they help us with a variety of functions. They help us digest our diverse diets, especially plant based foods which our body cannot break down. Our microbiome helps us absorb nutrients, synthesize vitamins, and break down products into short chain fatty acids (SCFAs) that are vital for health. For instance, butyrate is produced by bacteria breaking down fiber in your colon. Butyrate is the preferred food of cells in your colon; without it, they cannot maintain their normal function as easily and are more likely to become cancerous.
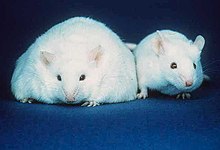
Transferring the microbiome of the fat mouse to the skinny one caused it to become fat. Picture from Wikipedia
Metabolism
Being colonized by the right bacteria helps people maintain healthier weights, and starting life with the right bacteria makes that easier. Germ-free mice become obese when they are inoculated with the microbiomes of obese mice. Children who are born vaginally are less likely to be obese than those who are delivered via Caesarian section, partially because the vaginal microbes help inoculate the baby with the proper bacteria. In a separate study, children who were inoculated with the probiotic Lactobacillus rhamnosus at birth were less likely to be obese than those who were not.
Immune function and the hygiene hypothesis
The microbiome helps the immune system develop and mature, building up its ‘memory banks’ to distinguish between harmless and harmful bacteria and environmental exposures (especially when we are young). Just like we need to build up tolerance to comfortably eat spicy foods, drink bitter beer, and build immunity to poisons, the immune system needs a gentle introduction to microbes to figure out what it can tolerate. With too little early exposure to bacteria (or without a properly developed microbiome), the immune system may be unused to handling things we encounter on a daily basis (or even our own bodies). This can cause it to expend huge amounts of energy attacking otherwise harmless substances (like peanuts, pollen, or our own cells), potentially causing anaphylactic shock.

Results of an allergy test. Yikes! Source: Wikimedia commons
Protection
The microbiome helps fight off invading bacteria, including pathogens that make you sick. Microbes can release toxins that kill or damage invaders. They can also enhance host immunity, recruiting immune system cells to attack outside organisms. Furthermore, by simply existing, your helpful microbes occupy space that could otherwise be colonized by harmful bacteria, preventing them from taking over.
Maintenance
So how can you maintain your microbiome? Help it along by eating probiotics like yogurt and fermented food that contain ‘healthy’ bacteria. You can also eat prebiotics; foods that provide nutrients to help certain types of bacteria grow. Scientists haven’t reached a consensus about the best ways to go about this, but research is ongoing to figure it out.
You should also be careful of how you treat yourself when you get sick. While antibiotics are necessary to fight off many infections, they are often requested for illnesses (like colds) against which they are ineffective. This unnecessarily damages the microbiome and can lead to higher antibiotic resistance overall. Furthermore, we haven’t been able to come up with an antibiotic that only attacks the pathogenic bacteria, so taking them always unbalances your microbiome by killing off portions of it. There is always a trade-off.

More like the 90%! Source
With ongoing research on the microbiome, we’ll learn more about how our microbial fellow-travelers have shaped us, and how we continue to shape them. For much of human existence, we’ve been
unintentionally changing our microbiomes with our shifting diets, medicine, and lifestyles. As we start understanding the impact we are having on our bacteria buddies, that era is rapidly coming to an end.
References
Guarner, Francisco, and Juan-R. Malagelada. “Gut flora in health and disease.”The Lancet 361.9356 (2003): 512-519. DOI
Bultman, Scott J. “Emerging roles of the microbiome in cancer.”Carcinogenesis 35.2 (2014): 249-255. DOI
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006). DOI
Ajslev, T. A., Andersen, C. S., Gamborg, M., Sorensen, T. I. & Jess, T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int. J. Obes. 35, 522–529 (2011). DOI
Darmasseelane, Karthik, et al. “Mode of delivery and offspring body mass index, overweight and obesity in adult life: a systematic review and meta-analysis.” PloS one 9.2 (2014): e87896. DOI
Cho, Ilseung, and Martin J. Blaser. “The human microbiome: at the interface of health and disease.” Nature Reviews Genetics 13.4 (2012): 260-270. DOI
Proal, Amy D., Paul J. Albert, and Trevor G. Marshall. “The human microbiome and autoimmunity.” Current opinion in rheumatology 25.2 (2013): 234-240. DOI
Gao, Zhancheng, et al. “Human Pharyngeal Microbiome May Play a Protective Role in Respiratory Tract Infections.” Genomics, proteomics & bioinformatics(2014). DOI
Morrow, Lee E., and Marin H. Kollef. “Probiotics in the intensive care unit: why controversies and confusion abound.” Crit Care 12.3 (2008): 160. DOI

